Curriculum 'endoACRO - Site User Training'
4. Apply for and create user account Download PDF
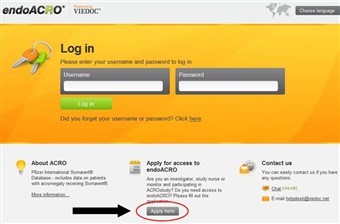
1 Application form
- To apply for a user account in endoACRO, please go to: www.endoACRO.com
- Open the application form by clicking the "Apply here" button.
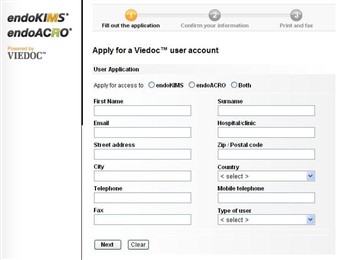
2 Fill out application
- Step 1. Fill out the application and click "Next".
- Step 2. Verify the information you entered and click "Next".
- Step 3. Print the application form, sign and fax it to +46 18 444 40 23 or scan and email it to helpdesk@viedoc.net
- Please observe that you cannot access the production study until you have been approved in the system by a Pfizer Monitor.
- By signing this document all electronic signatures you create in this system will be legally binding and equivalent to a traditional handwritten signature.
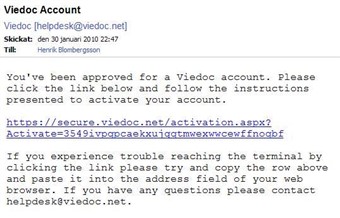
3 Activation email
- Once your application has been approved you will receive an activation email from helpdesk@viedoc.net.
- Click the attached link and enter the activation password. The activation password should have been provided to you by your local Pfizer contact. If you need help send an email to endoacro@pfizer.com
- Important! If you do not receive the activation email, please check your email spam folder.
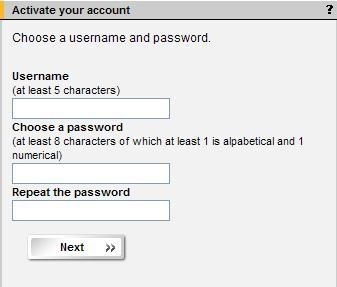
4 Create user account
- Choose a unique user name and a password according to the instructions. Click "Next".
- Your user account has now been activated.
- When you have activated your user account you will have access to the training study and can start training there.
- When you consider yourself fully trained and are approved in the system by a Pfizer monitor you can access the production study.
- Note! You can select a challenge question and answer in your user profile. The challenge question can be used to reset the password if you forget it. Contact helpdesk if you need support.
5 If you have a user account in endoKIMS
- If you already have a user account in endoKIMS you still need to apply for an account in endoACRO and fax or email you signature form to helpdesk, but the same account will be used.
- Once your application has been approved by helpdesk you will receive access to the training study. You will receive an email with information and a link to the endoACRO site.
- When you are approved in the system by the Pfizer monitor you will get access to the production study.