Curriculum 'endoACRO - Site User Training'
22. Exit and Re-entry Download PDF
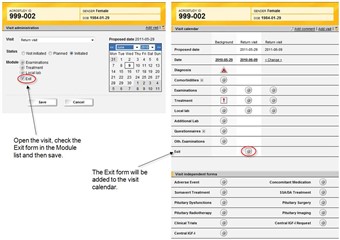
1 Exit
- Any patient may be discontinued from the study at any time at the discretion of the investigator or if it is the wish of the patient.
- To remove a patient from endoACRO you add the Exit form when initiating a new visit (by selecting it under Modules for the visit).
- The Exit form has to be signed for any effect to take place.
- Note! To delete an unsaved form added by mistake, go to Visit administration and click on the red symbol to the right of the form. Please contact PCG helpdesk if you have entered data in an Exit form by mistake.
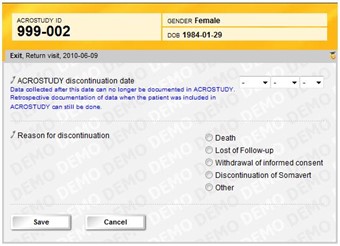
2 Exit options
- There are four exit options: Death, Lost of follow-up, Withdrawal of informed consent and Discontinuation of Somavert.
- If the patient died: AE pages should be filled out. If a Serious Adverse Event Death is reported the Exit form is automatically added to the visit calendar and should be filled out.
- Withdrawal of informed consent: all patient data is blinded for Pfizer.
3 Re-entry
- Re-entry is possible for any exited patients when initiating a new visit and selecting the Re-entry form under Modules for the visit.
- If the patient has withdrawn the informed consent a new informed consent must be obtained.
- The Re-entry form has to be signed for any effect to take place.