Curriculum 'endoACRO - Site User Training'
21. Central MRI reading Download PDF
1 ACROSTUDY Central MR Reading Instructions
- According to the ACROSTUDY protocol, if there is a change in tumour size after ACROSTUDY enrollment all images should be sent for central evaluation. Such evaluations are offered free of charge to all participating sites and performed by Prof. Dr. med. M. Buchfelder at the Neurochirurgische Klinik der Universität Erlangen-Nürnberg in Erlangen, Germany. The following points are essential to avoid confusion and to allow a proper interpretation of the MR investigations (MRI).
- 1. All MRI for evaluation should be sent directly from the ACROSTUDY site to the central evaluation center to prevent Pfizer from accessing patient personal information.
- 2. Please print out the ACROSTUDY Central MRI Reading form from the endoACRO system and fill in the information as relevant. Please note that the list of MRIs on the form is pre-populated based on the data entered to endoACRO in the pituitary imaging eCRF. Should certain MRI be missing, please go back to the pituitary imaging eCRF and check whether all required information is entered. If not, please complete data entry. MRIs and form should be sent together to the following address:
Prof. Dr. M. Buchfelder,
Tumor Volume Reference Center - Project ACROSTUDY-,
Neurochirurgische Klinik der Universität Erlangen-Nürnberg,
Schwabachanlage 6, 91054 Erlangen,
Germany.
Please, remember to fill in the center contact details. - 3. Either CD-ROM data sets or printed images on paper or film may be submitted for re-analysis. Particularly sagittal and coronal sections of T1-weighted images, preferably after contrast application, are appreciated. Please clearly mark the envelope by writing “ACROSTUDY” so that data and films can be identified as ACROSTUDY materials.
- 4. To obtain the best possible information on tumour size evolution, as many MRIs as possible should be submitted. Therefore send at least one MRI from before the start of Somavert therapy and as many MRIs as possible after Somavert start. After evaluation, the best possible fit sections of magnification, contrast and grey scale corrected images will be sent back to the investigator. The ACROSTUDY center will be alerted by email once a written report is uploaded and accessible at endoACRO. The reports will also be available to ACROSTUDY Medical Outcomes at Pfizer.
- 5. Original materials will be returned to the investigators within two weeks. The central evaluation center will only keep the most relevant images blinded for patient name. Should there be any queries, please do not hesitate to contact Mrs Silke Speck Neurochirurgische Klinik der Universität Erlangen-Nürnberg in Erlangen, Germany
- Phone : +49-9131-85-34566
Email: nch-sekretariat@uk-erlangen.de
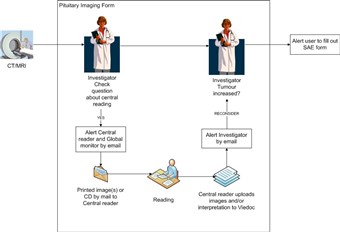
2 Central reading process in Viedoc
- Central reading can only be performed as a follow-up to Somavert treatment and the central reader will need to see images from before and at Somavert start for comparison.
- You should therefore start by adding entries for CT/MRI scans performed before and at start of Somavert treatment for a new patient. When images are sent to central reading you need to attach the media from previous scans and always enclose the first image taken and an image from the start of somavert treatment.
- When adding a new entry to the Pituitary Imaging log form in Viedoc you will first set if it is taken before, at or as a follow-up to Somavert treatment.
- If the CT/MRI scan is a follow-up to Somavert treatment there should be treatment recorded in the Somavert treatment log form in Viedoc.
- The Investigator makes a initial assessment of whether the tumour has increased since last examination.
- If the CT/MRI is a follow-up the media should be sent to central reading. Otherwise enter an explanation.
- If central reading is to be performed an automatic email alert is sent to the Central reader and the Global monitor.
- When the central reading has been performed the central reader uploads a report and image in Viedoc.
- An automatic email alert is sent to all investigators at the center.
- The investigator can now view the uploaded images in the Pituitary Imaging form and based on them set if the tumour has increased relative to ACROSTUDY start and previous examination.
(Note that the Investigator can only view, not edit, the files uploaded by the Central reader)