Curriculum 'endoACRO - Site User Training'
12. Adverse Event / Serious Adverse Event Download PDF
1 Add an Adverse Event (AE)
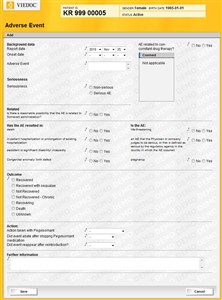
- Click the Adverse Event log form for the selected patient. Click the "Add" button to add a new event. Fill out the form.
- If the event is serious select the Serious AE option and the form will be extended with more questions. When you save a serious adverse event (SAE) a SAE report will be generated and you will be directed to the Documents page so that you can view the report. See more information below.
- If a SAE death is reported for a patient, the Exit form will be added to the Visit calendar and you should create an exit with reason death for the patient.
2 Copy adverse event
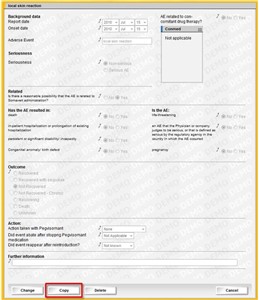
- If an adverse event is similar to a previously added adverse event you can simply open the old event and copy it by clicking the "Copy" button.
- Note! Remember to change the copied row to reflect the new event.
3 Serious Adverse Event (SAE) report
- The SAE report is generated automatically when saving a SAE or the AE is a pregnancy. The system redirects to the Patient documents area on the Documents page, where the report is highlighted. The report is pre-filled with patient data available in the Adverse Event form as well as other forms in Viedoc.
- Print out the report, fill in any missing data and send it to the Pfizer local Safety Department according to process described in the lesson "Serious Adverse Event Flow".
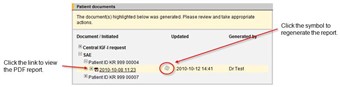
- To view the SAE reports generated for the patients at the center, click on the [Documents] menu link. In the Patient documents area there is a list of the patients which have SAE reports.
- The SAE report is regenerated automatically if you change anything in the SAE and then save it.
- If data has been changed in other forms included in the SAE report, for example Concomitant medication, the report should be regenerated to reflect the changes. Regenerate the report from the Documents page by clicking the regenerate symbol.
- If there are several versions of the SAE report there will be a plus sign in front of the PDF link. To view the different versions of the SAE report you click the plus sign.